A new stability analytics capability for the Matrix Gemini LIMS from Autoscribe Informatics features an integrated charting module providing early indication of shelf-life performance for each product or batch of product under test.
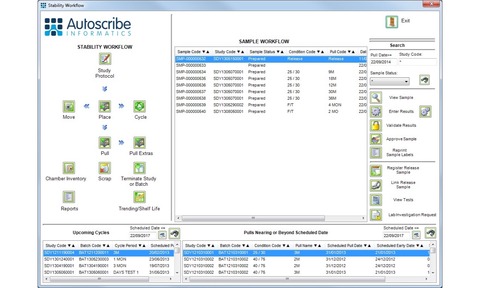
Stability studies are used to assess the shelf life of a product by storing samples under differing conditions, typically as defined by regulatory authorities, and then testing samples taken from storage at pre-determined intervals. Matrix Gemini LIMS is designed to help simplify the complex and time consuming whole study management process. The in-built stability study management functionality is designed to automate and control the entire operation of the stability study including protocol creation, study initiation and management, inventory management, sample pull points, future workload reporting and stability study reporting.
The system helps to optimise the number of samples stored for a study to avoid shortages and eliminating waste. Samples to be pulled are automatically registered with relevant tests and test limits assigned, and users are automatically alerted that the samples need to be removed from the storage environments. It is also possible to define and manage the stability chambers and track their contents. Recognising that no two organisations are likely to work in the same way, configuration tools allow specific workflows to be modified while maintaining the underlying stability functionality.
The latest stability analytics module provides an integrated system for analysing stability studies that involve multiple batches, users, and data transfers. Statistics and charting capabilities include ANCOVA statistics, tests for pool-ability, out of trend detection and residuals analysis. Predictions can be made using pooled data, pooled slope and worst case.
Simon Wood, Product Manager at Autoscribe Informatics, said: “The new software module further extends the Stability functionality present in Matrix Gemini. It closes the loop from set up and management of stability studies to the full reporting of the outcomes. Matrix Gemini manages the full lifecycle of stability studies and provides the support required to meet regulatory requirements including GMP and the FDA 21 CFR Part 11 regulations.”




