Copley Scientific will be displaying smart Facemask Testing Apparatus for metered dose inhaler (MDI) testing, at Drug Delivery to the Lungs 25 (DDL 25) in Edinburgh, Scotland, next month.
The company will be showcasing its full range of products to improve the effectiveness and efficiency of inhaled product testing at the Edinburgh International Conference Centre from 10th to 12th December, including the NGI Component Carrying and Rinsing Rack, which streamlines post analysis workflow.
MDIs provide inexpensive and effective relief from asthma and other pulmonary diseases, but can be difficult for certain patient groups to use, especially children and the elderly.
The use of spacers and valved holding chambers (VHCs) eliminates the need to coordinate inhalation with actuation of the device, making MDIs easier to operate successfully.
Draft USP chapter <1602>, which was released at the beginning of 2014, specifically addresses the testing of MDIs with spacers and VHCs and calls for the testing of facemasks, where they are used, to reflect clinical practice.
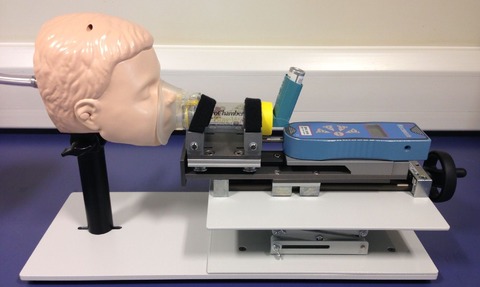
The Facemask Testing Apparatus from Copley Scientific comprises a range of angle-adjustable face models with replaceable skins, a slide rail mechanism and integrated load cell for precise, calibrated control of the applied force when advancing the facemask against the face model.
All components are mounted on a base plate using quick release fittings and the apparatus as a whole interfaces, using the appropriate filter holder and adapters, to the Copley range of breath simulators.
It is also designed to accommodate a wide range of spacer and VHC designs.
The face models used (baby, child and adult variants available) are based on the internationally recognised Laerdal geometries, but the system can be purchased and adapted by the user, for use with other face models if required.
Significant consideration has been given both to the relevance and ease of testing and Copley Scientific is looking forward to gathering initial customer feedback at DDL.




