Drug "may partially relieve" spinal injury
3 Dec 2014
Scientists in the US have developed a drug that could lead to a revolutionary new class of spinal cord injury treatments.
In laboratory tests, paralysed rats have exhibited partially restored axon growth and improved movements and bladder functions when injected with a drug called ISP, a US research team has claimed.
For seven weeks, the research team injected the group of rats with either the drug or a placebo near the site of a spinal injury.
“This is a great step towards identifying a novel agent for helping people recover
NIH programme director Lyn Jakeman
After several weeks, the rats that received the drug showed improvements in walking and urinating while the placebo treatments had no effect. The results suggested the drug passed into the brain and spinal cord, the researchers said.
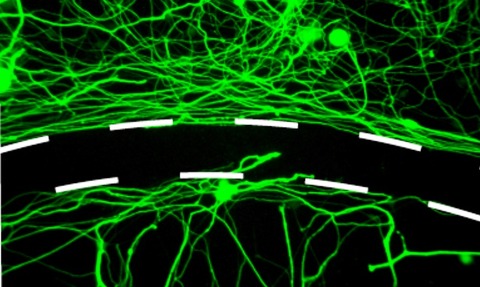
Spinal injuries can crush and sever the long axons of spinal cord nerve cells, blocking communication between the brain and the body and resulting in paralysis below the injury.
After spinal cord injury, axons try to cross the injury site and reconnect with other cells but are stymied by scarring that forms after the injury, the researchers said.
To combat this, Bradley Lang, lead author of the study, suggested the idea of designing a drug that would help axons regenerate without having to touch the healing spinal cord.
Lang and his colleagues designed ISP to block the protein tyrosine phosphatase sigma (PTP sigma), an enzyme found in axons, and facilitate ISP’s entry into the brain and spinal cord of the paralysed rats.
“There are currently no drug therapies available that improve the very limited natural recovery from spinal cord injuries that patients experience,” said Lyn Jakeman, a programme director at the National Institutes of Health (NIH), who partially funded the research.
“This is a great step towards identifying a novel agent for helping people recover,” Jakeman said.
Industry response to this announcement has been positive, though commentators have pointed to unanswered questions within the research.
“These findings are extremely important and will be of significant interest to the public, since this is a therapy that could potentially be fast-tracked to the clinic and could have the potential to help restore function to spinal injured patients, if the further research that is now needed supports the findings of this study,” said Elizabeth Bradbury, of King’s College London.
“Several questions do still need to be addressed, such as finding out why all of the animals did not respond to the treatment,” she said.
“This could be an issue of permeability of the peptide (i.e. how easily it enters the spinal cord tissue); the peptide may not have effectively entered the spinal cord tissue in the ’non-responders’.
“I am hopeful that this will be resolvable with further work.”
Likewise, Dusko Ilic, reader in stem cell science at King’s College London, said: “I hope that other scientists will explore this exciting development.”
The research team is now hoping to conduct preclinical trials, with the aim of developing a new class of drugs that could treat severe spinal injuries.
A full account of the study has been in the journal Nature.

