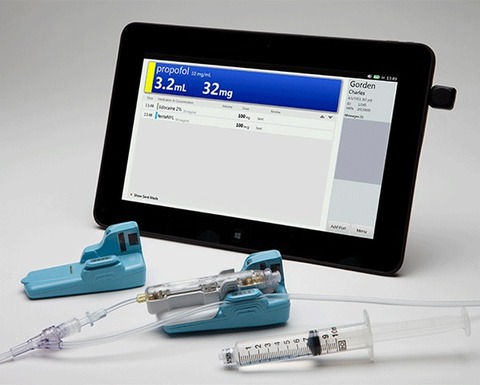
Titan Enterprises has been working with CRISI Medical Systems to develop a unique disposable flow sensor.
The company assisted CRISI in the initial development of the sensor used in the BD Intelliport, a medication management system for manual intravenous (IV) bolus injections.
After testing an Atrato ultrasonic flowmeter, CRISI took the basic design and - with help from Titan - miniaturised the assembly and made it into a disposable unit.
Titan has granted exclusive global rights to BD Intelliport for use of its Atrato technology in IV bolus injections.
BD Intelliport and CRISI then entered into an exclusive partnership to jointly develop the BD Intelliport Medication Management System for manual IV bolus injections.
The Intelliport system is claimed to be the first and only solution to provide real-time drug identification, dose measurement and allergy detection at the point of injection, while wirelessly sending captured information directly into the patient’s electronic medical record (EMR) following medication administration.
The Intelliport system received clearance from the US Food and Drug Administration (FDA) in December 2014 and is expected to be commercially available shortly.
Titan Enterprises’ Atrato ultrasonic flowmeter is a true inline non-invasive flow meter without the contorted flow path and disadvantages of alternative designs.
It can handle flows from laminar to turbulent so is largely immune from viscosity.
Available in 60°C and 110°C temperature versions and a 30 bar higher pressure model, Atrato flowmeters use patented ’time-of-flight’ ultrasonic technology that enable operation over very wide flow ranges (200:1) with excellent accuracy (better than ±1.0% of reading).





