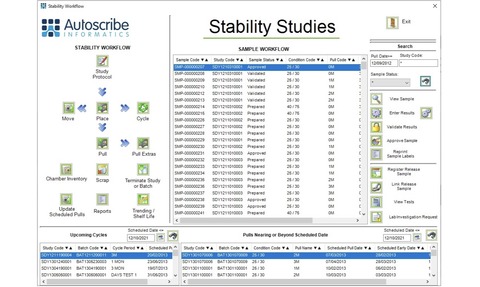
Matrix Stability, Autoscribe Informatics’ software solution for managing stability and shelf-life studies is to be demonstrated at the Stability VI Symposia hosted by the Royal Society of Chemistry (RSC) in London.
The system can be used to create stability protocols that define all required stability study attributes including pull schedules, cycles, storage conditions and test requirements for specific batches. Stability studies can also be automatically linked to product batches that have been evaluated using Autoscribe’s Matrix Manufacturing Solution.
Focussed on the management and assessment of drug stability, Stability VI is organised by the Joint Pharmaceutical Analysis Group (JPAG) and is jointly sponsored by the Royal Pharmaceutical Society and the Royal Society of Chemistry. Attendees will learn about the practical issues and challenges faced in managing stability work, and the basics of method development and testing.
Stability testing requires the management of significant amounts of data from a variety of sources. Starting with the automatic generation of 1D and 2D bar-coded labels, Matrix Stability optimises sample storage and management to avoid shortages and eliminate waste. Samples to be pulled are automatically registered with relevant tests and test limits assigned. Users are automatically alerted when samples need to be removed from the storage locations and tested. The system also provides security and audit trail functionality which complies with regulatory requirements including GMP and 21 CFR Part 11. Suitable for in-house or contract operations, the system can create quotes and generate sophisticated reports around storage chamber capacity.
Matrix Stability comes with standard forms which can create the necessary documentation for the stability protocol and its associated results. It is also possible to generate projected shelf-life trend analysis from the stored test results. The statistics and charting capabilities include ANCOVA statistics, tests for poolability, out-of-trend detection and residuals analysis. Predictions can be made using pooled, pooled slope, and worst-case data.
Simon Wood, Product Manager at Autoscribe Informatics, said: “Matrix Stability provides a solid foundation for drug stability studies in the pharmaceutical industry. The scheduling functions vastly simplify and automate the management of stability programmes and ensure critical pull points are never missed. Creating industry-standard shelf-life statistics from the test results recorded within the built in LIMS functionality allows entire study programmes to be managed by a single system. Matrix Stability manages the full lifecycle of stability studies and provides the support required to meet regulatory requirements including GMP and the FDA 21 CFR Part 11.”
Further information about the Stability VI Symposia can be found at: https://www.jpag.org/?p=meetings&r=155
Further information on Matrix stability is available at: https://www.autoscribeinformatics.com/lims/stability




