The benefits of using a Laboratory Information Management System (LIMS) to capture and manage data, optimise operations and eliminate wastage are summarised in a new webinar.
‘Using LIMS Data Analytics to Drive Laboratory Efficiency and Quality’ highlights typical KPIs needed in a laboratory, including Turn Around Time (TAT), workload, efficiency, and cost. It also shows how Matrix Gemini LIMS can capture and present this data, including performance analysis using Statistical Quality Control (SQC) charts.
Effective laboratory data analytics requires all relevant metadata to be captured as the sample moves through the laboratory process. The educational video emphasises the importance of identifying, acquiring, and storing this, using LIMS to keep an audit trail of all the changes and actions that have occurred. Data from instrument calibration, competency management, and Corrective Actions and Preventive Actions (CAPA) modules, among others also provide valuable additional sources of information.
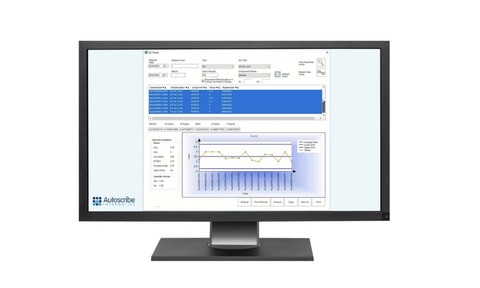
Example KPI screens on the webinar demonstrate sample throughput, instrument loading, and client specific TATs. Sample and management reports are very often specific to laboratories, so it is important to be able to easily adapt reports.
Statistical Quality Control (SQC) charts built into Matrix Gemini LIMS quickly perform statistical analysis and sample test results, enabling variances and drift to be quickly identified. A QC control chart was demonstrated as an example for attendees. How bracketing can be implemented to take account of both process limits and results of QC samples was discussed, as was automatic calculation of process limits based on previous results.
Marketing manager Tim Daniels said: “Autoscribe Informatics regularly holds educational webinars for the laboratory industry but our webinar on data analytics broke all records for registration and attendance. It demonstrates that laboratories are hungry for information on how they can best make use of their data to optimise operations, both within their laboratory and across the Company as a whole.”
A video of this webinar can be accessed here.




