UCL Advanced Diagnostics (UCL-AD) has chosen Roche’s HER2 assays to advance its programme of companion diagnostics for cancer patients.
UCL-AD is the immunohistochemistry, in situ and molecular diagnostics service arm of the UCL-Cancer Institute and a bridge between the Institute and University College Hospitals NHS Trust (UCLH).
It conducts around 110,000 immunohistochemistry, 1000 in situ and 1000 molecular diagnostic tests per year for a whole spectrum of diseases. It is also the host laboratory for the UK National External Quality Assessment Service for immunocytochemistry and in situ hybridisation.
Michael Gandy, Lead BMS & Clinical Services Manager at UCL-AD explained: “We have been evaluating a range of different HER2 targeted assays which are used as aids in selecting breast and gastric cancer patients who may benefit from Herceptin and next generation targeted therapies.
“Following an internal evaluation over a six month period we have chosen the HER2 4B5 and Inform HER2 Dual ISH (DDISH) assays on the Benchmark ULTRA staining platform from Roche as we see this technology as a major innovation.”
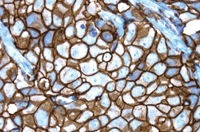
UCL-AD was seeking additional benefits from adopting new technology and chose the Roche solution because of the accuracy of the test and the benefits of using ‘brightfield microscopy’.
The test also enables a greater understanding of HER2 gene status in the context of the pathological characteristics of the disease, which is a major advantage in accurate targeted therapy selection, and delivers dramatically improved turnaround times through full laboratory automation.
Keith Miller, Chief Scientific Officer at UCL-AD said: “We are always cautious of adopting new technology, but we believe that having trialed HER2 DDISH over a prolonged period, we understand the subtleties of the test and are confident of the benefits of this brightfield technology.”




