Shedding light on scattering theory
18 Apr 2013
Malvern’s Paul Clarke takes a look at how light scattering theory can improve detector choice.
Gel permeation chromatography, also known as size exclusion chromatography (GPC/SEC), is heavily relied upon for its ability to measure properties, such as molecular weight, that define the performance of polymers, macromolecules and proteins.
The success of the analysis is strongly dependent on the selection of an application-appropriate detector array, with light scattering technology a popular option. However, making a selection is complicated by the commercial availability of a number of different detector technologies.
Light scattering detectors are valued because of their ability to directly measure molecular weight without the need for a closely similar standard. This makes it possible to apply GPC/SEC reliably to new materials or those which are less well-characterised.
With a little light scattering theory behind them, anyone looking to purchase a detector will be able to ask the right questions
Furthermore, in combination with other detectors, light scattering instruments enhance the productivity of analysis. For example, used with a viscometer, a light scattering detector can provide information about structure and branching, as well as dependable measurements of hydrodynamic diameter.
All light scattering detectors work on the same measurement principle, which for the dilute solutions typical of GPC/SEC applications is described by this simplified version of Rayleigh’s equation:
R?=0 = KcMw
Where: R?=0 is scattering at zero degrees to the incident beam, K is a constant, c is concentration and Mw is weight average molecular weight.
This mathematical correlation, the only one I will be introducing, is central to any discussion of the relative merits of different detector technologies. The key thing to notice is that it relates molecular weight very specifically to the value of scattering at zero degrees to the incident beam.
Measuring scattering at this point is physically impossible, because the magnitude of the scattering signal is dwarfed by that of the incident beam. Different technologies have therefore been developed to provide practical solutions to this problem; these are successful to varying degrees for specific applications.
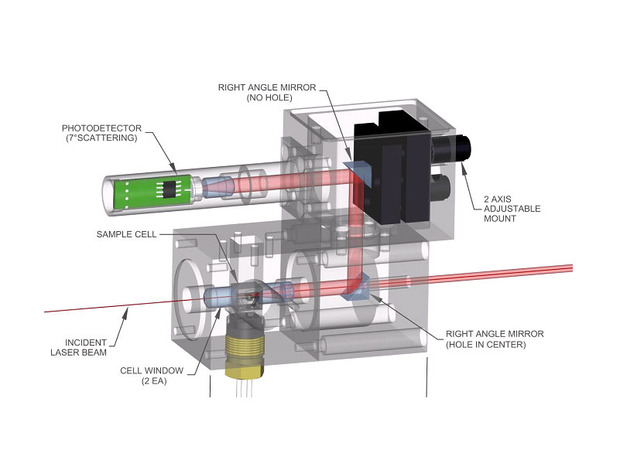
Low angle light scattering (LALS) detectors measure at an angle of 7o using a smart optical design developed to provide a physical solution to the underlying problem. This angle is so close to the incident beam that measurements are essentially identical to the value of scattering at zero degrees, avoiding any requirement to extrapolate, for any type of sample.
The fact that no mathematical correction or extrapolation is applied to the data supports and explains the claim that LALS detectors directly measure absolute molecular weight.
By detecting scattering at 90o to the incident beam, right angle light scattering (RALS) detectors maximise signal to noise ratio thereby enhancing the accuracy of measurement. However, they provide accurate values for the scattering at 90 o, not 0o, which brings us to the question of how the two are related.
For some molecules, typically those less than about 12 nm in size, the relationship is a simple one since scattering is isotropic - the same intensity is seen at every angle. The majority of proteins fall into this category along with some other sample types like low molecular weight polymers, and here RALS is a sound choice.
For larger molecules that scatter anisotropically, though, RALS is clearly less appropriate and LALS or MALS must be used.
Multi angle light scattering (MALS) detectors measure scattering at a number of angles to profile any anisotropic scattering behaviour and enable extrapolation back to the 0o figure required. So, they rely on a mathematical workaround, rather than a physical solution, to address the underlying problem.
Unfortunately anisotropic scattering is neither predictable nor uniform, from molecule type to molecule type. Therefore the accuracy of this approach depends on the choice of extrapolation fit and the number of detectors at lower angles.
MALS enjoys widespread use, and has one benefit, as the extrapolation exercise applied for the molecular weight calculation also leads to the generation of radius of gyration data which can be important for certain polymer applications. But this size measurement only works for large molecules and not for small species like proteins.
On the other hand it could be argued that MALS is sometimes used for applications because of established industry practice, where in fact there are smarter and simpler solutions available that could provide better quality data.
My hope is that with a little light scattering theory behind them, anyone looking to purchase a detector will be able to ask the right questions to help identify the best option for their needs and consequently to secure the optimum data integrity for all their samples.
Bio:
Paul Clarke is a graduate of the UK Royal Society of Chemistry. He completed his studies at Kingston University in 1985. After working in gas and liquid chromatography as a government chemist, he entered the instruments industry specialising in HPLC and GPC. He has now worked in the field of multi-detection GPC/SEC for over 20 years, and is product group manager for Nanoparticle and Molecular Characterisation at Malvern Instruments in the UK.