Making the right connections
8 Aug 2013
Dr Bülent Peker of Olympus microscopy explored how researchers at the Institute of Neurosciences, Technical University Munich have been studying the neuronal connections within the health and disease sectors.
The research is motivated primarily by axon damage associated with multiple sclerosis (MS). Misgeld and his team desired to assess the axonal interconnections between various parts of the nervous system.
Establishing the correct neuronal wiring is fundamental throughout brain development and during the continued adaptation of our neural circuits to environmental stimuli.
During this process, axons not only need to find and connect to the correct target, but incorrectly connected axons must also be removed.
“The research is motivated by axon damage associated with multiple sclerosis
Common neurological diseases such as MS and spinal cord injury can be characterised by early loss of axonal connections.
Real-time in vivo observations are vital for understanding the axon behaviour underlying these conditions and that is why studying live neurones in mice using high-resolution microscopy is considered to be so important.
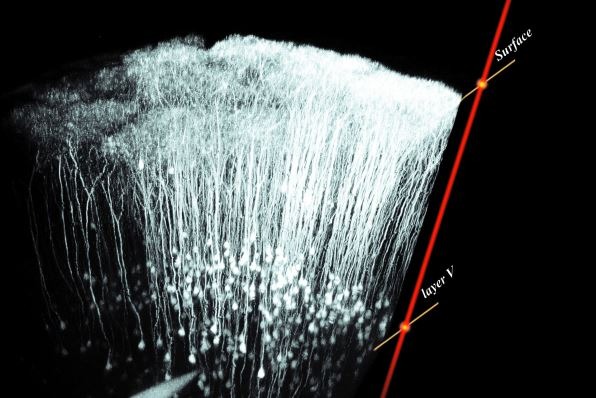
Two such methods used to image axons within mouse and zebrafish models are confocal laser scanning microscopy (CLSM) and multiphoton excitation (MPE) microscopy. For example, MPE was used to capture the above 3D image, showing neuronal layer V in mouse cerebral cortex in vivo, taken with the Olympus FluoView FV1000.
To facilitate the development of new treatments, these techniques help researchers dismantle and understand the nature of axons to yield insight into the fundamental causes of disease.
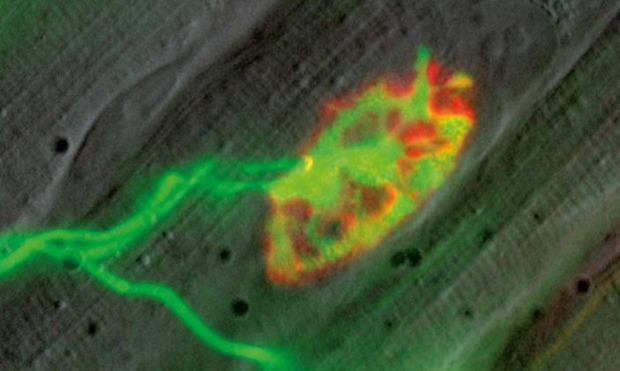
In collaboration with Professor Martin Kerschensteiner, the Misgeld group developed a transgenic mouse model, genetically labelling neurones with fluorescent proteins expressed under the control of a modified Thy-1 promoter element.
Real-time in vivo microscopy is a highly useful tool for investigating the behaviour of live axons within the Thy-1-/- mouse model and both CLSM and MPE are two techniques ideally suited to such studies.
Focusing on a single defined focal plane, CLSM is able to exclude this secondary fluorescence, achieving superior resolution. Moreover, the ability to obtain optical sections also enabled unobstructed analysis of intricate neuronal structures.
For in vivo studies, however, the excitation technique employed in standard CLSM can hamper time-lapse live cell imaging through phototoxicity.
To overcome this limitation, MPE technology uses two or more photons of long-wave infrared light, diminishing out-of-focus phototoxicity and photon scattering throughout the sample.
Protecting live neuronal samples from harm enables extended time-lapse observation of many processes, including the decomposition of an axon - hence the use of an Olympus FluoView FV1000MPE multiphoton microscope.
Long-wave light of this nature also permits fluorescence imaging of deep sample regions down to several hundred micrometres, allowing visualisation of the finest branching of neuronal axons within a living organism.
The microscopy system employed by Misgeld and his collaborators was modular in design. This allowed equipment to be added where necessary which enabled specialised recording of ultra-fast reactions with no time delay or loss of data.
Recording axonal branching in such detail has facilitated the study of neurodegenerative diseases and Misgeld’s team hope to further its research to achieve results and bring treatment from the bench to the bedside.
To download a copy of ’Making the right connections’, please click on the link below.