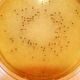
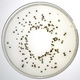
Lab M's neutralising media can help determine the effectiveness of hygiene procedures or can help determine the bactericidal capability of a specific disinfectant.
Neutralising media are used for the isolation and recovery of micro-organisms from surfaces treated with disinfectants.
These media neutralise the bactericidal and/or bacteriostatic activity of the residual disinfectant, allowing any contaminating organisms to grow.
Easy to prepare, Lab M's neutralising media range includes a variety of different formulations.
Letheen Broth is used to assess the bactericidal activity of preservatives in water-based cosmetics.
Letheen Agar enables determination of the number of colony-forming units from a hygiene swabbing protocol and, when used in conjunction with Letheen Broth, avoids potential inaccuracies caused by disinfectant carryover.
Microbial Content Test Agar (MCTA) is recommended for the detection of micro-organisms on surfaces sanitised with quaternary ammonium compounds, phenolic compounds and formalin.
D/E Neutralising Agar can be used when assessing disinfectants by the disc-diffusion method, while D/E Neutralising Broth and D/E Neutralising Broth Base are both suited for challenge testing of disinfectants.
The inclusion of bromocresol purple in D/E Neutralising Broths permits a visual detection of microbial growth through a colour change in the broth.