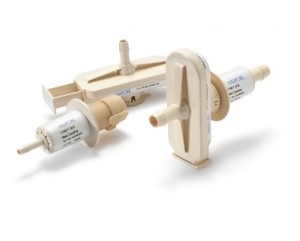
EMD Millipore has announced the release of two new sizes of its Lynx S2S connector, a single-use device for linking pre-sterilised fluid paths in biopharmaceutical processes.
The device facilitates secure connections in upstream and downstream processes as well as final fill and finish processes.
Its flexibility supports a broad range of applications, particularly sterile liquid transfer and microbiological sampling.
The Lynx S2S is the only connector to demonstrate a sterility assurance level of 10-6 under even the most rigorous conditions.
It is part of Millipore's Mobius line of flexible bioprocessing solutions.
The addition of these smaller 1/4in and 3/8in connectors to the Lynx S2S product line facilitates the connection of small diameter tubing.
All sizes are interchangeable, which allows for the connection of different sized tubing.
Millipore's high level of sterility assurance is based on the most stringent testing in the industry.
The test simulated worst-case environmental contamination by challenging the Lynx S2S connectors with greater than one million aerosolised Brevundimonas diminuta bacteria.
All Lynx S2S connectors passed this test.
The Lynx S2S connector's design allows fluid paths to be assembled in both classified and non-classified environments.
A sterile fluid path is enabled by connecting the device's male and female couplings.
The Lynx S2S connector is gamma compatible up to 45 Kilogray (kGy) and easy to validate.




