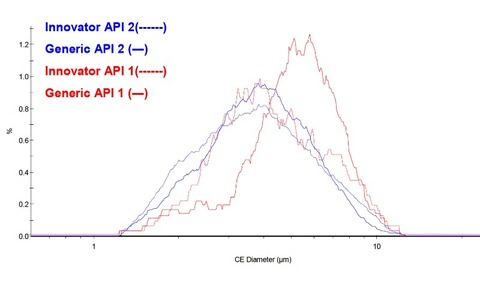
Malvern describes the use of morphologically directed Raman microscopy in assessing the bioequivalence of a generic and innovator drug.
The work was carried out using the Morphologi G3-ID, which combines automated image analysis with Raman spectroscopy.
The Morphologi G3-ID enables the determination of component-specific particle size distributions, which in this study were important because of the effects of particle size on tablet disintegration and subsequent bioavailability.
To download the report, please click on the link above.




