EKF Diagnostics has introduced a test to precisely measure levels of COVID19 neutralising antibodies in individuals.
The Kantaro COVID-SeroKlir SARS-CoV-2 IgG antibody test kit determines both the presence and specific quantities of human IgG antibodies to the SARS-CoV-2 virus. This enables a broad range of COVID-19 applications, such as delivering vital knowledge for advancing the understanding of protective immunity, assessing vaccine response and accelerating therapeutic treatments.
The high performance quantitative COVID-SeroKlir kit has received FDA Emergency Use Authorisation (EUA) and is CE marked. It has demonstrated 98.8% sensitivity and 99.6% specificity for detecting SARS-CoV-2 specific IgG antibodies against two SARS-CoV-2 virus antigens, the full-length spike protein and its receptor-binding domain (RBD). This confirmed accuracy means false positives and false negatives are minimised.
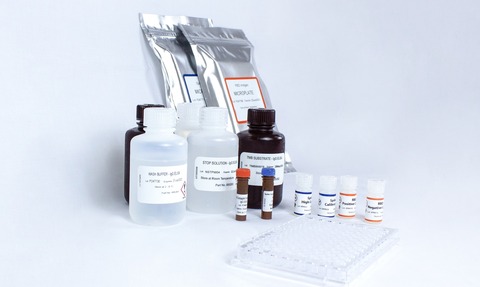
Being a two-step enzyme-linked immunosorbent assay (ELISA), ensures COVID-SeroKlir’s accuracy. The ELISA’s initial plate screens for RBD positive or negative samples, whilst the second plate provides a quantitative result of the antibody titre/concentration for the full-length spike protein. As the kit uses standard methods and equipment, it is easily operated without need for scaled equipment or special environments and contains components to test 630 patient samples.
The best-in-class serologic COVID-19 assay is based on technology developed by clinicians at the Icahn School of Medicine at Mount Sinai Health System in New York in partnership with RenalytixAI, a spinout company from EKF Diagnostics. It was validated on a highly diverse cohort of more than 75,000 patients, including more than 30,000 who were diagnosed with COVID-19. It was also independently verified by peer reviewed journals, including Nature and Science and the US National Institutes of Health (NIH).
The Mount Sinai study demonstrated that more than 90% of infected individuals with mild-to-moderate COVID-19 experience robust IgG antibody responses against the viral spike protein. These COVID-19 neutralising antibody levels were confirmed to be relatively stable during the first five months after infection.
Julian Baines, CEO of EKF, said: “Quantitative IgG antibody testing can provide important support for determining public health strategies, informing healthcare decision making, and verifying the effectiveness of vaccines as they become available. It is also an essential component of a general health check to determine past COVID-19 infections. This is because COVID-19 has been linked with an increased risk of potentially life-threateningcomplications, including lung, kidney, and cardiovascular disease.”
EKF holds exclusive rights to market and distribute the Kantaro COVID-SeroKlir kit in the UK and Germany, and non-exclusive rights in the rest of Europe. Baines added: “With the capacity to manufacture up to ten million tests per month, EKF is well placed to drive rapid availability of COVID-SeroKlir kits to a broad range of laboratories for immediate operation without needing specialised testing equipment.”