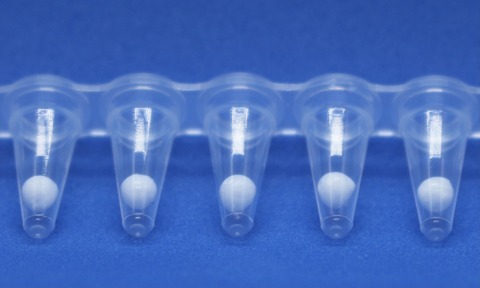
In response to demand, Biopharma Group has not only expanded its freeze-drying production capacity for pharma, diagnostics and vaccines, but also the drying methods used to include spray drying and lyobeads (freeze dryable spheres).
The move has proved invaluable to customers in the US, UK and Ireland, with the increased volume and speeds offered by employing lyobead technology proving particularly popular.
Biopharma Group scientists initially worked hard on the development of lyobead technology in response to the demands of Covid-19 for diagnostics and vaccines, but it is proving to be beneficial for many applications.
One of the most significant benefits is that it is not a standalone service. Biopharma Group’s dedicated team of lyo-scientists, ISO 13485 accreditation and R&D lab conduct supporting work to ensure that it is the most viable drying option for any given product - from proof-of-concept studies to formulation development and post-process analysis.
Lyobeads also offer significantly increased volume of product and efficiency when compared with conventional freeze-drying methods. Their formation means they require no aliquoting and no freeze-thaw cycles after constitution – helping to protect against batch variation and product degradation. Lyobeads are also highly adaptable and offer an impressive ratio of volume to surface area, which by return offers fast, efficient reconstitution results
Because lyo scientists can produce millions of reaction units a week and package them in a specifically controlled environment if required, lyobeads can contribute to improved ROI on an R&D investment. Discover more here.




