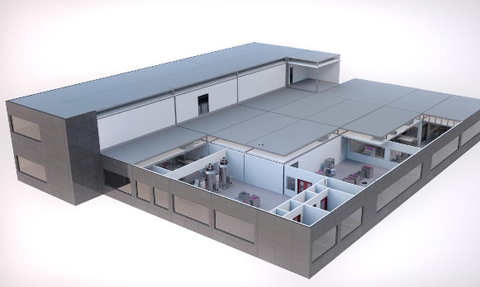
The KUBio is an off-the-shelf modular factory designed to save manufacturers of biopharmaceuticals time and money.
KUBio is a fully functional off-the-shelf bioprocessing facility specifically designed to meet cGMP requirements while optimising manufacturing flexibility and productivity.
The 1200m2 facility is pre-fabricated and delivered with a complete ready-to-use production line, based on GE Healthcare’s Ready-to-Process single-use technologies.
With a total planning, delivery and construction time of 14 - 18 months, compared to the traditional 24 - 36 months, both time-to-market and level of capital investment claim to be significantly reduced.
The first modular facilities to be introduced will be configured for the manufacture of monoclonal antibodies. GE Healthcare also plans to introduce modules for the manufacture of other biopharmaceuticals.
“KUBio is a totally new approach to the manufacture of biopharmaceuticals, bringing our customers a greatly simplified way to build their production capacity,” said Olivier Loeillot, General Manager of Enterprise Solutions, GE Healthcare Life Sciences.
“With KUBio our customers will be able to cut months off the typical construction time, and greatly streamline the whole construction process.”




